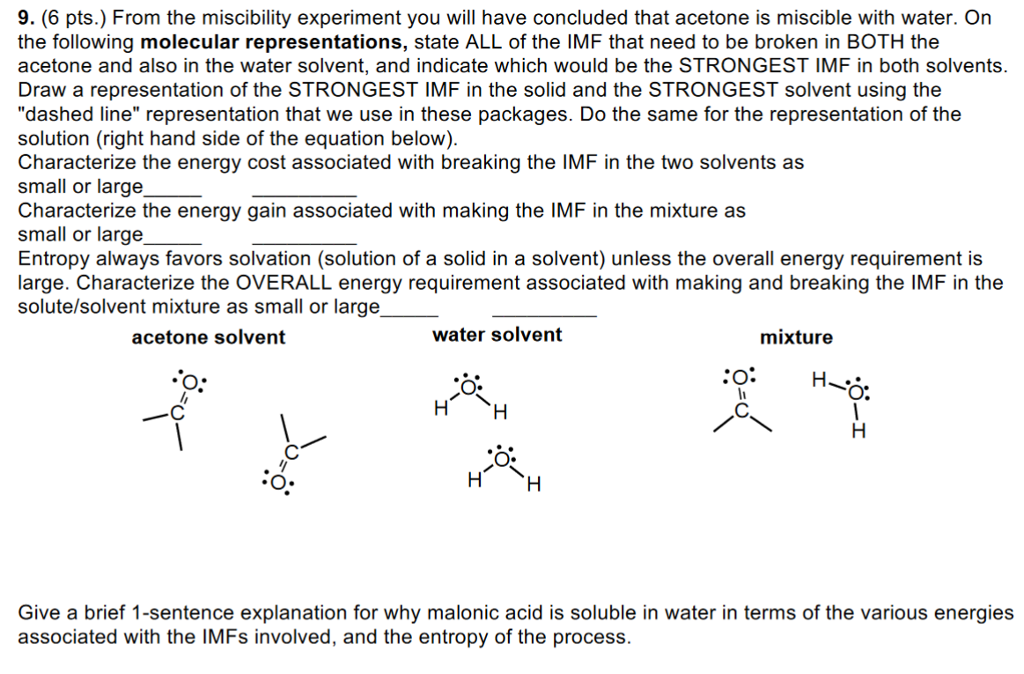
Separation of acetone: From a water miscible system to an efficient aqueous two-phase system - ScienceDirect

Acetone–water biphasic mixtures as solvents for ultrafast SET-LRP of hydrophobic acrylates - Polymer Chemistry (RSC Publishing) DOI:10.1039/C7PY00557A
Is a 50/50 mixture of acetone and water an azeotrope? Also, why does the first drop of destillate form at 40C? - Quora

Modeling of Mixing Acetone and Water: How Can Their Full Miscibility Be Reproduced in Computer Simulations? | The Journal of Physical Chemistry B
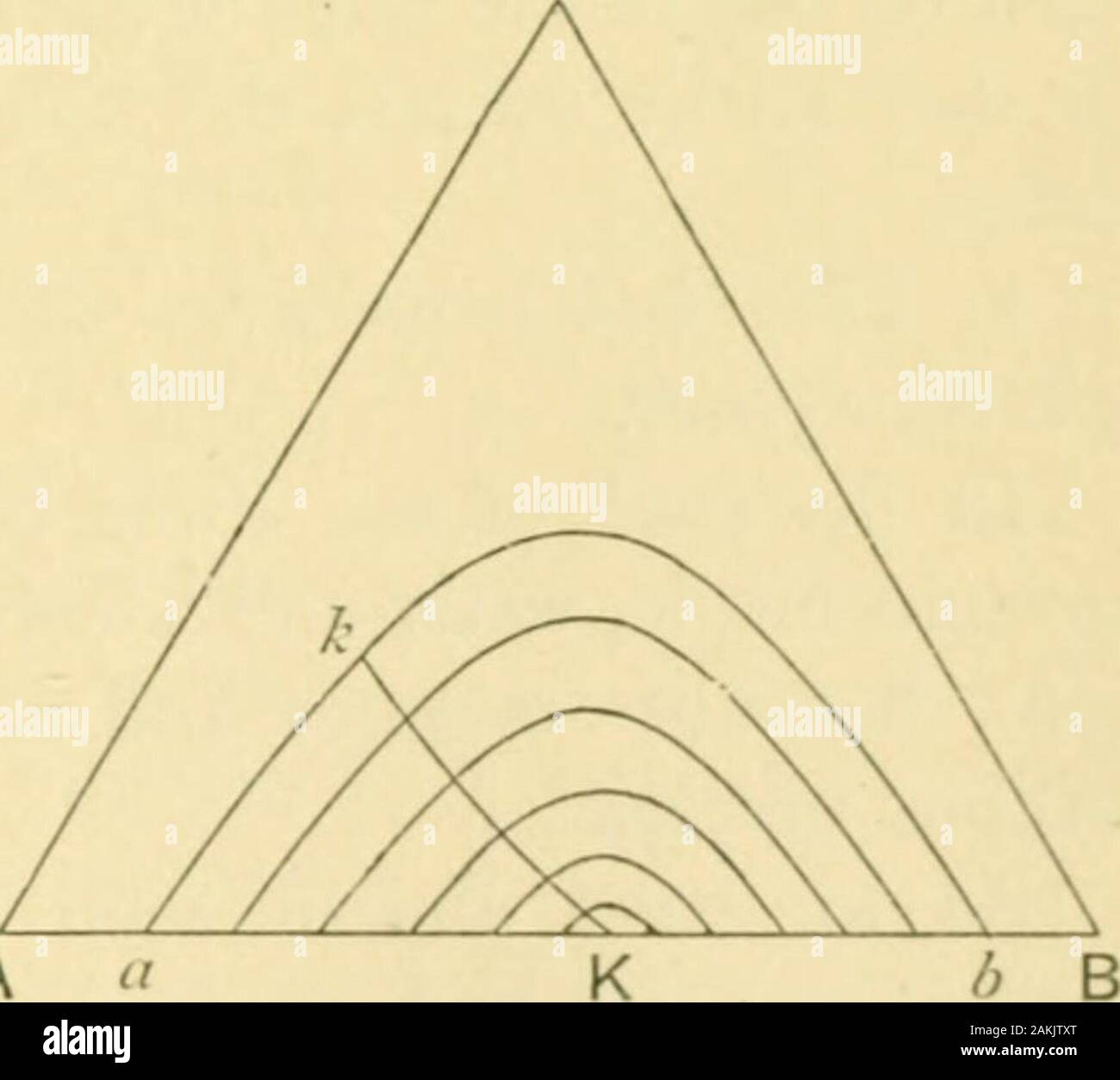
The phase rule and its applications . binary system, and K is the ternary critical point.All points outside the helmet-shaped boundary surface representhomogeneous ternary solutions, while all points within thesurface belong to

Separation of acetone: From a water miscible system to an efficient aqueous two-phase system - ScienceDirect

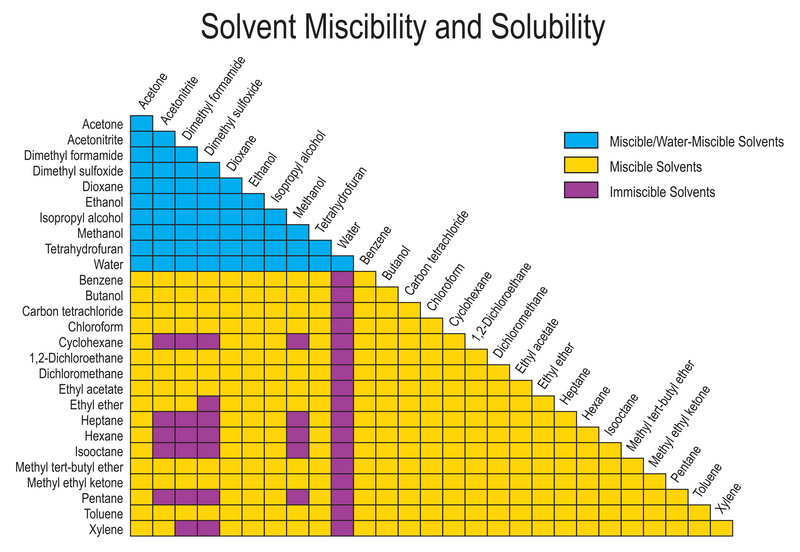
solutions - What determines solubility (or miscibility) in organic solvents? - Chemistry Stack Exchange

3. The systems acetone–sodium hydroxide–water and acetone–potassium hydroxide–water at 0° - Journal of the Chemical Society (Resumed) (RSC Publishing)

Crystal growth rates of paracetamol in mixtures of water + acetone + toluene - Granberg - 2005 - AIChE Journal - Wiley Online Library
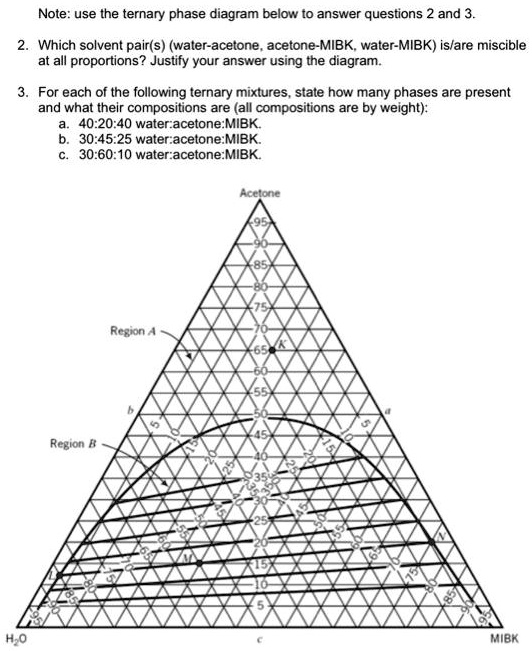
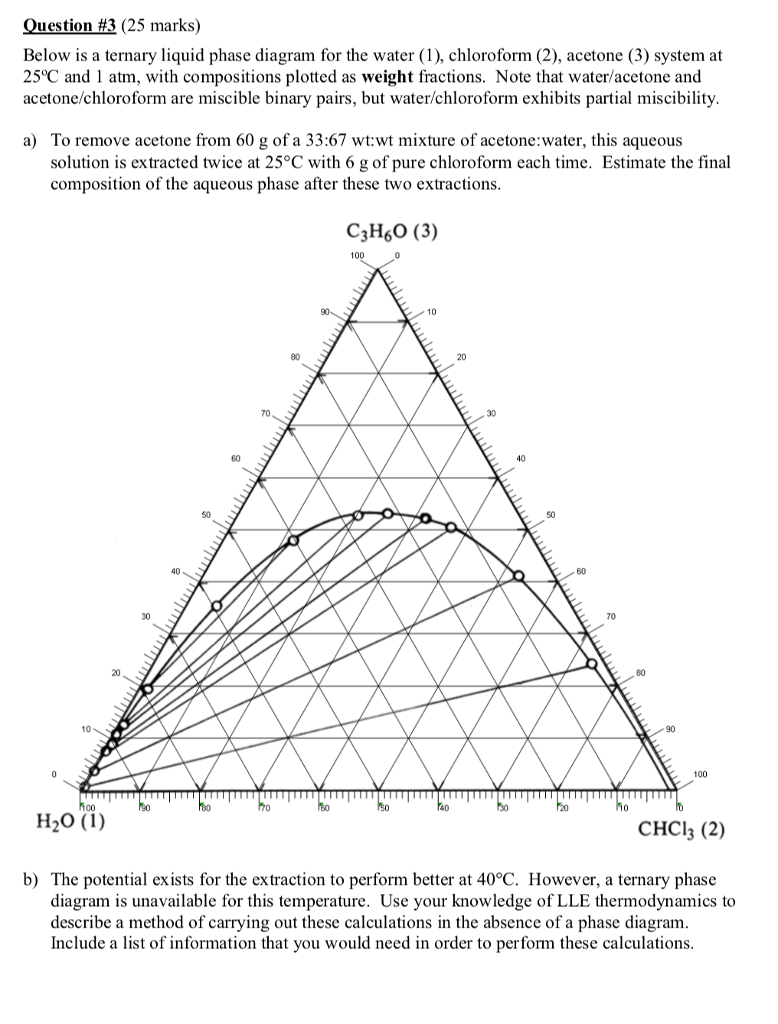
SOLVED: Note: Use the ternary phase diagram below to answer questions 2 and 3. 2. Which solvent pair(s) water-acetone, acetone-MIBK, water-MIBK is/are miscible? 3. For each of the following ternary mixtures, state

Modeling of Mixing Acetone and Water: How Can Their Full Miscibility Be Reproduced in Computer Simulations? | The Journal of Physical Chemistry B

Modeling of Mixing Acetone and Water: How Can Their Full Miscibility Be Reproduced in Computer Simulations? | The Journal of Physical Chemistry B

Polymers | Free Full-Text | Effect of Acetone as Co-Solvent on Fabrication of Polyacrylonitrile Ultrafiltration Membranes by Non-Solvent Induced Phase Separation
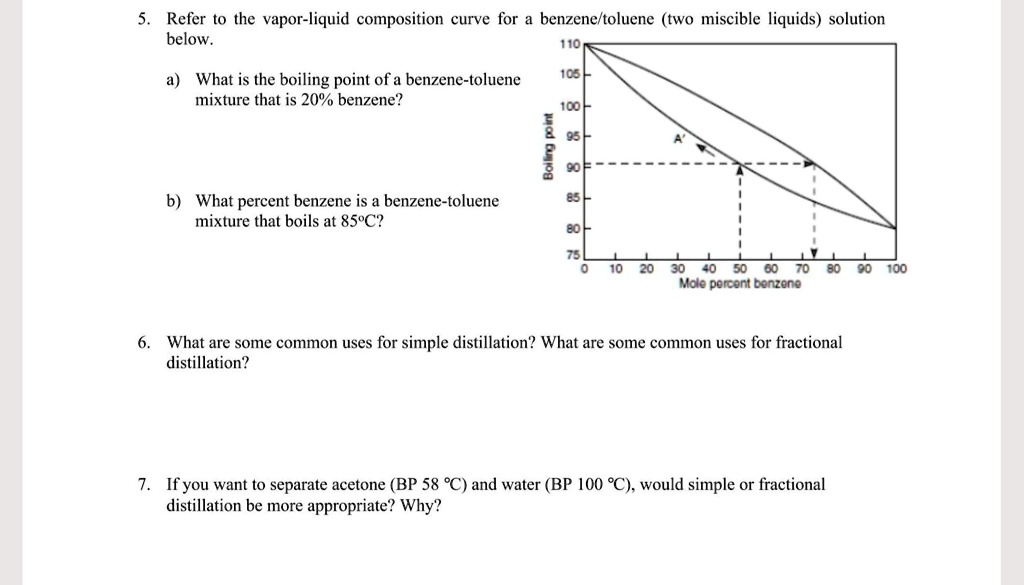
SOLVED: Refer to the vapor-liquid composition curve for a benzene/toluene (two miscible liquids) solution below. What is the boiling point of a benzene-toluene mixture that is 20% benzene? IC5 ICo 3 What